Individual COI
Definition and Scope
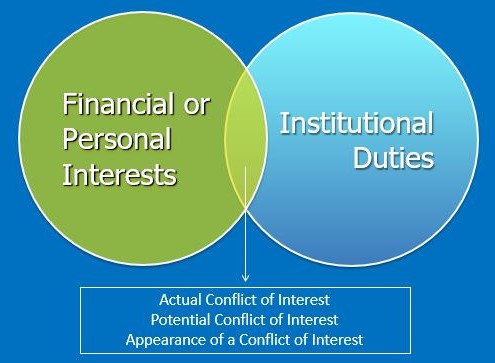
Conflict of interest relates to situations in which financial or other personal considerations may compromise, may involve the potential for compromising, or may have the appearance of compromising a Covered Individual’s objectivity in meeting University duties or responsibilities, including research activities.
The bias that such conflicts may impart can affect many University duties, including:
- decisions about personnel,
- the purchase of equipment and other supplies,
- the collection, analysis and interpretation of data,
- the sharing of research results,
- the choice of research protocols,
- the use of statistical methods,
- and the mentoring and judgment of student work.
Details about the information required for COI review are in the Policy on Individual Conflicts of Interest and Commitment.
All faculty, staff, students, trainees are considered Covered Individuals and are subject to the Individual COI Policy for their University duties and activities.
Individual COI may arise in a variety of situations, as detailed in the Policy. Some key areas:
- Research
- Intellectual Property Transactions
- Use of University Resources
- Gifts to the University for the individual’s benefit
- Gifts or Favors to Individuals
- University Review Panels
- Purchasing or business transactions
- Administrative Roles
Principles
It is the goal of the Conflict of Interest Office, along with any applicable COI Committees or applicable individuals, to manage any identified conflict in accordance with the following principles:
- Transparency
- Honoring the Student/Trainee Experience
- Protection of the credibility of the individual doing the work
Management
Management of any conflict is determined on a case by case review, specific to the situation. With transparency as essential to COI management, the required foundation is:
- Public disclosure of the relationships/conflict
- In any public dissemination, including but not limited to manuscripts, publications, presentations, abstracts, web page, activity sheets.
- In informed consent documents for any human study. The text is subject to approval by the reviewing IRB.
Other options include:
- Monitoring
- Alternative options for trainees
- Alternative administrative routing and/or recusal
For on-going relationships, a written management plan is sent to the individual with standard terms applicable to the scenario. This plan serves as the core ethics and foundation of the COI management. Additional terms are added as applicable in such cases as research studies (see Research section) or if new administrative duties arise.
Confidentiality
The information provided on a COI disclosure is considered part of an individual’s personnel file and is generally not subject to public records requests. Information will be shared with those who have a business need to know the information, such as the Institutional Review Board (IRB) or members of Conflict of Interest Committees.
For certain PHS-funded studies, other guidelines may also apply where the University is required to share the information upon request. Please see sections V. Research H.6(b) and VI. Records Confidentiality and Retention of the University’s Policy on Individual Conflicts of Interest and Commitment.
Disclosure Submission Troubleshooting
If you or any personnel experience technical issues within the AIR system, please call the Office of Research Information Systems (ORIS) help desk at 919-843-2594. Please be prepared to provide as much information as possible to expedite troubleshooting.
Please contact our office if you need help with:
- Answering any questions on the COI disclosure forms
- Correcting mistakes on a previously submitted COI disclosure
Consequences of Non-Disclosure
It is a requirement for individuals to disclosure their personal or financial interests as described in the Policy. The consequences of failing to disclose one’s interests, as they relate to one’s University duties, can vary depending on the circumstances. Please see section VIII. Policy Breaches of the University’s Policy on Individual Conflicts of Interest and Commitment.
With the size and breath of the University’s research portfolio, Section V.H of the Policy on Individual Conflicts of Interest and Commitment, Research and Sponsored Projects, is the most heavily used section along with its corresponding SOPs.
COI Study Specific Process
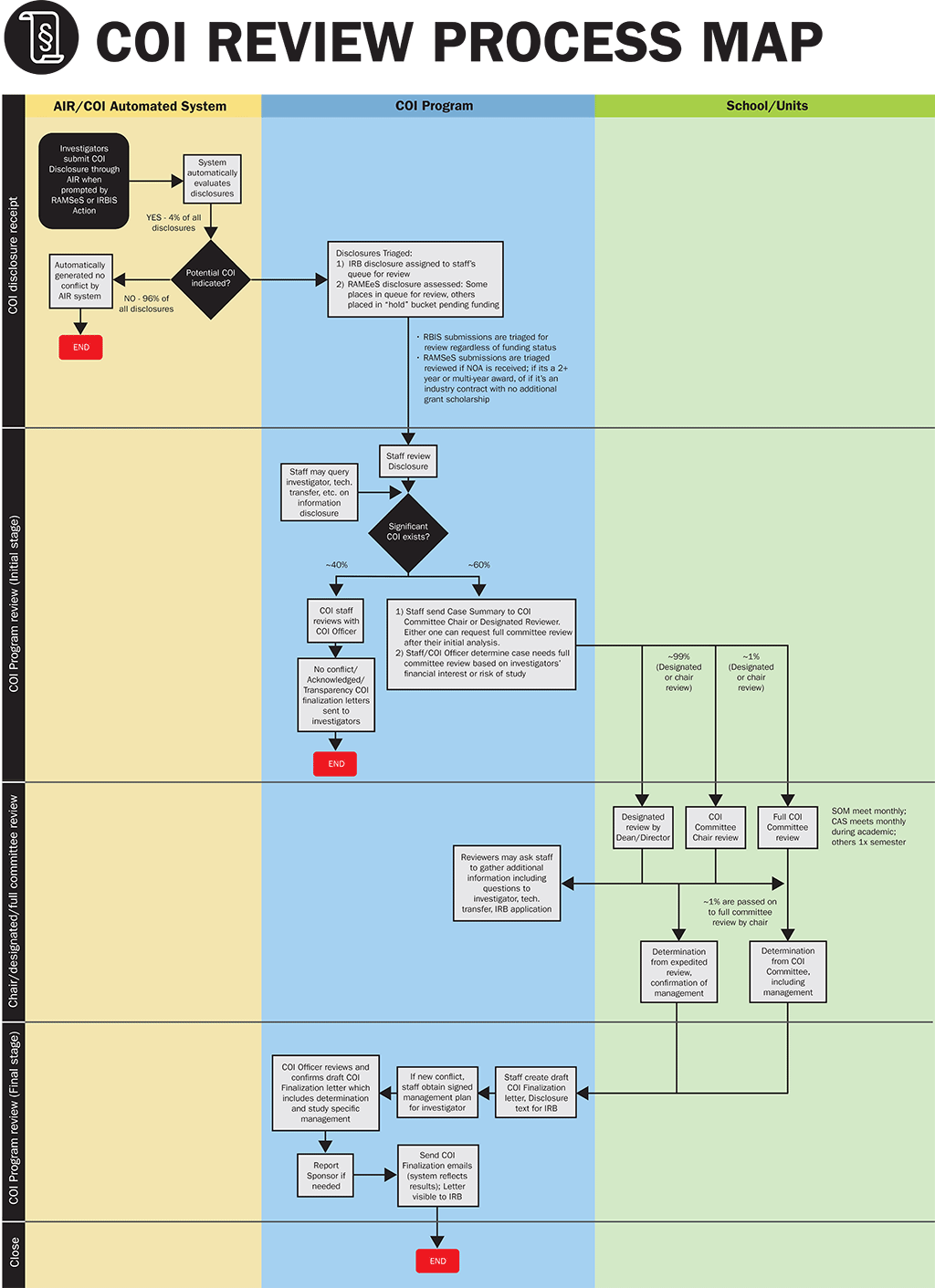
- Individuals receive email notification to complete a COI disclosure upon submission of an Office of Sponsored Programs (OSP) proposal (IPF) or IRB application (IRBIS).
- Individual answers the questions on COI disclosure form in order for disclosure to be received into the online AIR (Activities, Interests and Relationships) system.
- Once disclosure is received into the online AIR system, the form is assessed by the system for positive responses.
- If no conflicts are indicated, emails indicating no conflict are sent automatically.
- If information is included about a possible conflict, the form is triaged for review by the COI staff within the Conflict of Interest Office.
- Every disclosure starts with an Administrative Review. Depending on the nature of the potential conflict, the disclosure may go through additional reviews, starting with a COI Chair, the applicable COI Committee or a designated reviewer. Each disclosure is reviewed on a case-by-case basis.
- Determinations are returned for each individual. The status is reflected into the related research system such as IRBIS (human study) or RAMSeS (sponsored project).
Checking “yes” on a conflict of interest disclosure form does not necessarily put an investigator at risk of losing funding. Sometimes it is determined that no conflict exists. At other times the conflicts can be managed through a combination of oversight and disclosure.
Please note that individuals working in a research study might be required to complete several COI disclosures, each associated with different RAMSeS proposals and/or IRBIS applications. Per federal regulations (OSP) and accreditation requirements (OHRE), a COI review is required for each research study or project. Many RAMSeS projects can support multiple IRB applications. While, at the same time, IRB applications can have multiple funding sources in RAMSeS. Therefore a 1:1 relationship between the two systems is rare. While investigators might consider all of it one research study, the University considers the RAMSeS proposals and IRBIS applications independently, and thus more than one COI disclosure may be required for each individual working in a research study.
Automated Reminder Notifications
IRBIS and RAMSeS are configured to send out reminder email notifications once a study-specific disclosure has been initiated.
Checking Status of Individual Research COI reviews
- RAMSeS proposals and awards
- RAMSeS – Compliance Tab/Conflict of Interest (COI) Grid
- IRBIS applications
- IRBIS – Personnel Tab/Conflict of Interest (COI) Grid
Human Studies
Similar any any aspect related to human studies, there is a higher standard for any individual with conflicts to be involved in human studies. There is particular concern regarding the use of licensed intellectual property by inventors or when there is also equity interest held by the investigator which is related to the study or where the results of the study could benefit the company.
Participation as an investigator with a conflict would require at minimum meeting a threshold of Compelling Circumstances as detailed in the Policy. If compelling circumstances threshold is met, additional management requirements are usually determined, including separation from particular study activities. Transparency is the informed consent is a requirement and expected for any human subject study.
Determination Letters
COI determination letters are sent to individuals after the completion of the review, as detailed in the SOPs. If it’s a new conflict, a signed management plan must be obtained from the investigator before the COI determination letter can be sent out.
The COI finalization letter will contain a determination, an acknowledgement of existing management, or text indicating the need for new (or revised) management to be established. Any management terms, such as public disclosure, are included in the letter and are immediately applicable upon transmission to the Investigator via the email. Informed consent text for human studies is included in this letter and is subject to approval by the reviewing IRB.
When a COI Finalization email is sent, the status is reflected immediately in the related research system at OSP or the IRB.
COI Review Completion Required
In accordance with federal regulation and regulatory standards, COI review must be completed before any sponsored project is funded or before an IRB study can move to final review. Accordingly the Offices of Sponsored Programs and Human Research Ethics have “hard stops” built into their related online systems such that studies or projects cannot move forward until all COI disclosures have a completed status.
Intellectual Property Transactions
Prior to the technology transfer office, the inventors who are University Covered Individuals are required to submit a self-initiated COI form. The technology transfer office will provide directions to these individuals regarding specific information to include.
Purchasing, Contracting or Other Routine Business Transactions on Behalf of the University
Covered Individuals generally may not participate in awarding, negotiating, reviewing, or approving a financial transaction (including but not limited to purchases, contracts, and subcontracts) involving the University and an entity in which the Covered Individual has a Personal or Financial Interest without prior review and approval.
In accordance with NC State Statute 14-234, such transitions may also be prohibited.
Submitting a Self-Initiated Administrative Disclosure
To complete a Self-initiated COI disclosure, please go online to the Activities, Interests, and Relationships (AIR) management system. Click on the Self-Initiated Conflict of Interest disclosure menu item in the middle of the page. Please enter an appropriate title for the project, and select the sponsor (this is the entity with which you have a relationship to report). Then proceed to answer the rest of the questions. Please be sure to certify and submit the form when you are done.
Travel Disclosures
University individuals who have an Onyen can log into the AIR (Activities, Interests and Relationships) system. This system serves as a portal to manage all disclosure and request forms related to Conflicts of Interest (COI) and Conflicts of Commitment (COC). You may access any COI disclosure form through this website.
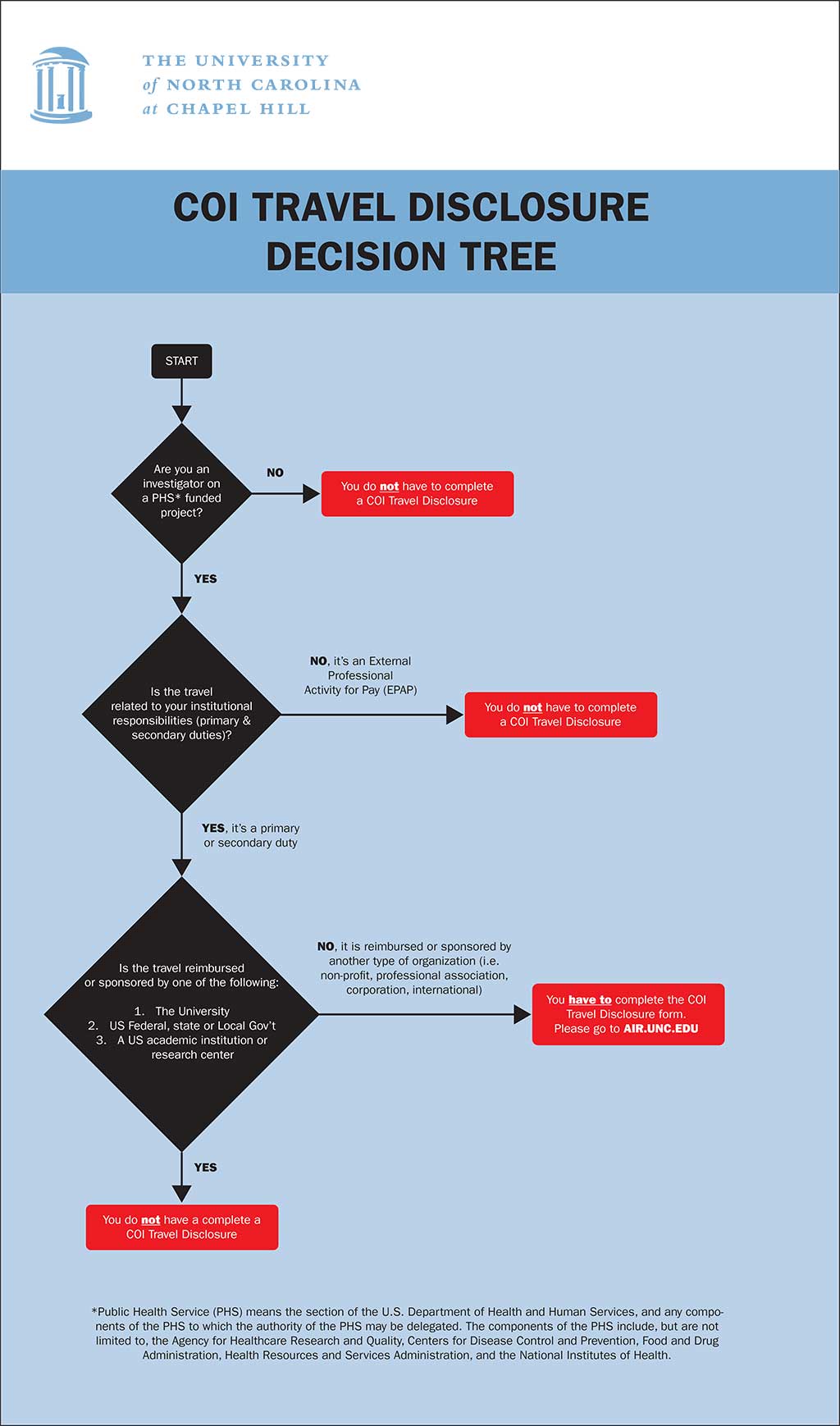
Travel disclosures can be submitted before the travel occurs. An individual can submit a disclosure up to 30 days after the travel.
Note: Federal Exclusions
All exclusions listed in federal regulations only apply to U.S. institutions of higher education or federal, state or local government agencies within the United States. Travel related to institutional responsibilities that is sponsored or reimbursed by universities outside of the United States, by a government of another country or by a non-governmental organization (NGO) must still be disclosed to the University. Please use the decision tree to determine if you need to disclose.
Travel Disclosure Decision Tree
Ethics Authorization Forms
For individuals who are traveling to a vendor sponsored event, some companies require the University to complete “government ethics authorization forms”, which are required for event attendance. The form should be submitted to the COI Office at least seven days prior to the deadline. In addition to the form, the following information must be provided:
- Does the individual make purchasing recommendations of the vendor or sponsor of the event?
- Does the individual have final signing or purchasing power for the vendor or product?
- Can a list with breakdown of specific amounts being given be provided?
Paid Authorship Forms
If you are PHS/NIH funded, you must disclose paid authorship. Please submit via the AIR website.
If you are considering or planning to assign a book to a class you are teaching where you could receive royalties (money) from the sale of the book, please use the Paid Authorship Form.
